
In other words, we could theoretically measure the speed of the fastball to thirty-two decimal places! The quantum uncertainty plays no role in this measurement. In matching units, the position uncertainty is 10 centimeters or 0.1 meters, the mass is about 0.3 kilograms, and h = 6.63 x 10 -34 Joule seconds. What is the quantum uncertainty in the speed of the baseball? Rearranging the equation above, the uncertainty in speed Δv = h / (4π x Mass x Δx). The radar gun measures its speed as it passes through the 10-centimeter beam of radar. Suppose that a pitcher throws a 90-mile-per-hour fastball (about 40 meters per second). Or we can know its motion, but at the cost of not knowing where it is! The amount of uncertainty is given by Planck's constant, a tiny number that has little or no effect in the everyday world. What does the mathematical form of the Heisenberg uncertainty principle mean? It means that we can know the position of a tiny particle, but only at the cost of not knowing its motion at all. The equation is an inequality, saying that the product of the uncertainty in the position and the uncertainty in the momentum is about equal to or greater than a very tiny number. In this equation, Δx is the uncertainty in the position in one direction, Δv is the uncertainty in the velocity in that same direction, and h is the universal constant named after the early 20th-century physicist Max Planck. Where the Greek letter Δ is the commonly used symbol for the error or uncertainty in a quantity. In the everyday world, this effect is too small to be noticed, but it dominates our understanding of the microscopic world. Thus, there is a fundamental uncertainty in the subatomic world: the act of measurement always alters the objects being measured. But at some point, the photon, which must have a long wavelength if it has a low energy, becomes too big to interact with the particle. We could be clever, and shine a photon of lower and lower energy at the particle. But the photon hitting the particle moves it, and we no longer know where the particle is! So our knowledge of its position comes at the expense of our knowledge of its motion. Now if we are looking for a tiny subatomic particle, we might try to illuminate it with a single photon. Light reflecting off the object gives it energy and so moves it slightly. Searching for tiny objects with a light beam, the energy and momentum of the photons become significant. But what about when we are looking for a tiny particle, instead of a ball? It turns out that we can no longer be sure of the position of the tiny object. As far as we are concerned, the flashlight lets us see the position of the ball as accurately as we want.

Energy state and time in heisenberg principle full#
In the macroscopic world full of big objects, this tiny amount of energy carried by photons is negligible. So when the photons bounce off the ball, which allows you see it, the ball gains a tiny amount of energy. However, light in the form of photons carries energy. A smarter way would be to search for it with a flashlight. How would you find it? You could blunder around in the dark, but it is very likely you would bump into it and send it off in some unknown direction.
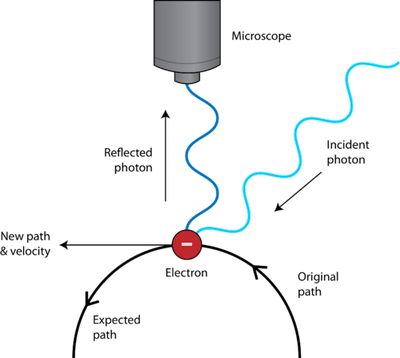
Imagine there is a ball somewhere in a completely darkened room.


 0 kommentar(er)
0 kommentar(er)
